Behind the paper: Nakayama, K., Ishikawa, R., Kobayashi, S., Shibata, N. & Ikuhara, Y. Dislocation and oxygen-release driven delithiation in Li2MnO3, Nature Communications (2020).
Lithium-ion secondary batteries are the most familiar and essential energy storages for consumer electronics. As shown in Fig. 1a, lithium-ions move between the cathode and anode during a charging or discharging process. So far, to improve energy capacities of lithium-ion secondary batteries, plenty of efforts have been devoted to exploring cathode materials with higher lithium contents. The lithium-excess layered oxides of Li2MnO3 and the relatives of Li(4-x)/3TM(2-2x)/3TMxO2 (TM = Ni, Co, etc.) are now recognized to be promising materials owing to their high lithium contents (Fig. 1b). The theoretical capacity (up to 460 mAh g-1) is 1.6 times higher than that of LiCoO2 (280 mAh g-1), where LiCoO2 is the current standard material for lithium-ion batteries. In practice, however, the lithium-excess oxides show severe degradations during charge and discharge cycles, which is significant especially in the first or second cycles. To address this degradation issue, it requires detailed analyses of the delithiation process at atomic level.

Fig. 1 a Schematic view of the lithium-ion secondary battery in a charge process. b Crystal structures of current standard Li2CoO2 (left) and lithium-excess Li2MnO3 (right). Li2MnO3 is characterized by the additional lithium sites in the transition metal layers, as indicated by the arrows. These excess lithium-ions contribute to the higher energy capacities.
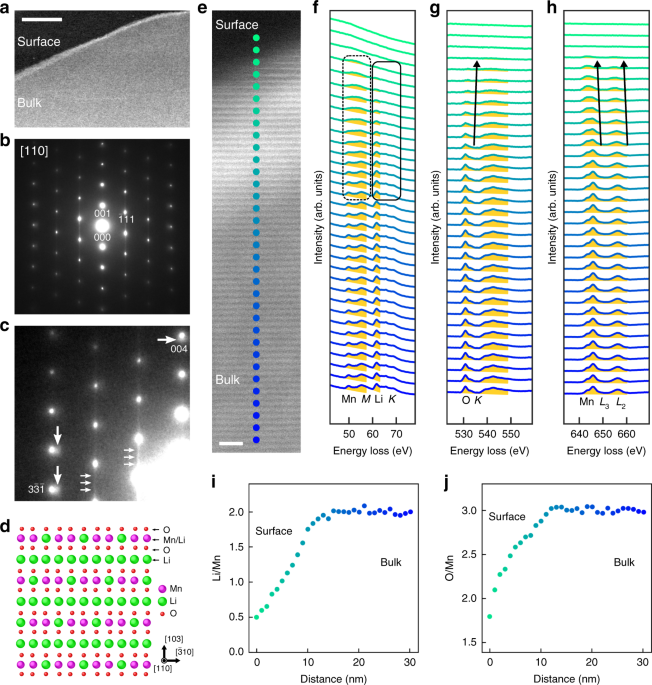
The delithiation process for respective grains of an active material should be proceeded by the growth of the delithiated region as show in Fig. 2a. This growth is governed by the motion of the interface between the delithiated and pristine regions, and thus the interfacial structure is the key to understanding of the delithiation process. In this work, we prepared partially delithiated Li2MnO3 single crystal and investigated the interfacial atomic structure by using scanning transmission electron microscopy (STEM) combined with electron energy-loss spectroscopy (EELS) (see the schematic views in Fig. 2b – d).

Fig. 2 a Schematic view of the delithiation process of a Li2MnO3 grain. The delithiation proceeds along with the motion of the interface between the delithiated and pristine regions. b Schematic view of the sample preparation for atomic-resolution STEM imaging and spectroscopy. A single Li2MnO3 crystal was partially delithiated from the surface, and electron-transparent thin samples for STEM were made by a focused ion beam (FIB) technique. c Schematic view of STEM. By scanning a focused electron probe (less than 0.1 nm) over a thin sample and detecting the transmitted or diffracted electrons, atomically resolved structural images are obtained. There are mainly two imaging modes, namely, the annular bright-field (ABF) and annular dark-field (ADF). With the aid of the electron energy-loss spectroscopy (EELS), we can also analyze chemical compositions and electronic structures. d Photo of our microscope (ARM200CF, JEOL Ltd.).
With the aid of EELS analysis, we found that oxygen in Li2MnO3 is partially released during the delithiation process, because the tetravalent Mn is hard to be further oxidized. On the close inspection of atomic structural analysis in the delithiated region, we found that Mn atoms are partially substituted for the Li sites, leading to the formation of Mn/Li disordered structure. At the interface between the delithiated and pristine regions, we found the cation disordered new structure. In addition, dislocations (one-dimensional lattice defects) were also found at the interface (Fig. 3a, b). Such dislocations are introduced to compensate the lattice mismatch between the expanded delithiated region and the pristine region, where the lattice expansion is induced by oxygen-release.

Fig. 3 a ABF-STEM image of the delithiated sample, where the dark dotted contrasts correspond to the locations of atomic columns. Dislocations were found at the interface between the delithiated and pristine regions, as well as the delithiated region. b Strain map obtained from the ABF-STEM image in a, where the large local strains confirm the presence of dislocations. c Schematic view of the delithiation process accompanied by the oxygen-release and climb motion of dislocations.
On the basis of the atomic-resolution observations, the delithiation process of Li2MnO3 is given as follows (Fig. 3c). First, lithium-ions are extracted from the surface, and simultaneously the oxygen-release occurs. As a result, the lattice is expanded, and dislocations are introduced from the surface to compensate the lattice mismatch. Lithium and oxygen atoms in the inner pristine region diffuse respectively into lithium- and oxygen-vacancies in the delithiated region through the delithiated/pristine interface (from inside to the surface), and the interface moves toward the opposite direction (from the surface to the inside). The delithiated region grows by repeating these processes, accompanied by the climb motion of dislocations.
In short, the delithiation process of Li2MnO3 progresses simultaneously with the climb motion of dislocations arising from the local lattice expansion due to the oxygen-release. Since the oxygen-release should be irreversible, the delithiation process should also be irreversible. This proposes that the creation of dislocations would contribute to the performance degradation of energy density and durability. In other words, the battery performance would be improved by suppressing the creation of dislocations, which may be achieved by adding suitable elements to suppress the oxygen-release or controlling grain size and shape to relax strains. We will be happy if our findings are considered when designing new better batteries with higher capacities and long cycle life.
For more information, please see our paper:






Please sign in or register for FREE
If you are a registered user on Research Communities by Springer Nature, please sign in